 Image credit: © 2021 Elsevier Ltd.
Image credit: © 2021 Elsevier Ltd.
Abstract
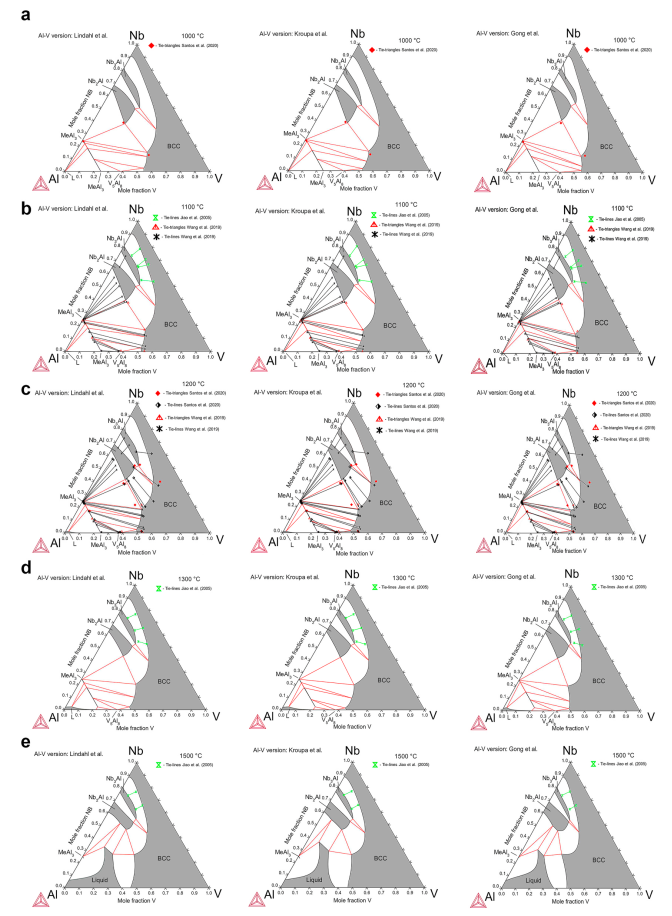
In the present work, the Al–Nb–V ternary system was thermodynamically modeled using the compound energy formalism (CEF) within the framework of the CALPHAD method and supported by ab initio calculations, which we report here for the first time. The thermodynamic descriptions of the Al–Nb, Al–V and Nb–V binary systems were taken from literature. Since it is not clear which is the best Al–V assessment available in the literature, three sets of thermodynamic parameters with three different thermodynamic descriptions of the Al–V system are proposed. End-member energies of Nb3Al and Nb2Al were obtained from first-principles electronic-structure calculations following the density functional theory (DFT). The optimized ternary parameters were based on experimental isothermal sections and liquidus projections data. The three sets of thermodynamic parameters show good agreement with experimental data. The new assessments successfully describe the miscibility gap between NbAl3 and VAl3 close to the Al–Nb system, the V solubility in Nb3Al, the tie-triangles Nb2Al-NbAl3-BCC and NbAl3-BCC-V5Al8, and the tie-lines between Nb3Al and BCC. In this sense, our work provides self-consistent thermodynamic descriptions of the Al–Nb–V system which can be used for the development of thermodynamic databases of higher order systems for refractory high-entropy alloys (RHEAs).